Abstract
Background. Based on the IFM 2009 trial, lenalidomide, bortezomib, dexamethasone (RVD) induction regimen, followed by autologous stem-cell transplantation has been the cornerstone of the treatment for newly diagnosed, transplant eligible multiple myeloma (MM) patients for many years. The treatment has been used because it allows for achievement of a deep remission in the majority of patients before stem cell collection which has been shown to be of benefit for the prognosis of patients. While this is a standard approach to induction, there are many variations in dosing. Two of the most common schedules are an RVD-21 (lenalidomide days 1-14, bortezomib days 1,4,8,11) and an RVD-28 (lenalidomide days 1-21, bortezomib weekly for three weeks). Despite the broad use of this regimen in myeloma therapy, there has been limited data that compare the efficacy and safety of these variations of RVD. This is a retrospective analysis of patients treated at a single center that compares the safety and efficacy of both schedules in newly diagnosed multiple myeloma.
Methods. Patients were assigned into two groups based on the prescribed treatment schedule: RVD-21 or RVD-28. The primary endpoint of this study is overall survival (OS). Secondary endpoints include progression-free survival (PFS) at first relapse (PFS-1) and at second relapse (PFS-2) and time to very good partial response (VGPR). Safety outcomes are evaluated via the incidence and severity of adverse events. OS and PFS were summarized by variables of interest using standard Kaplan-Meier methods. Multivariable Cox regression was performed to adjust for potential confounding variables. VGPR or better response rates and adverse events between two groups were compared using Fisher's exact test.
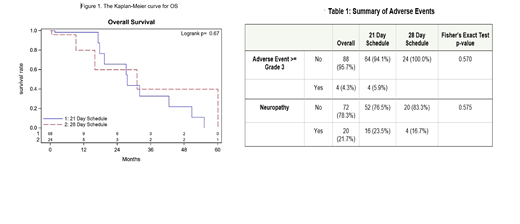
Results. A total of 92 patients were included in the analysis, 67 patients were started on RVD-21 and 25 on RVD-28. Four patients were switched from RVD-21 to RVD-28, and 2 patients were switched from RVD-28 to RVD-21. The median follow-up was 2.8 months. No difference was found in OS between the two RVD schedules in the univariate analysis, with median survival of 27.6 months in the RVD-21 and 31.1 months in RVD-28 (P=0.668) (Figure 1). Median PFS-1 (C1D1 to death or first progression) observed was 35.1 months in patients on RVD-21 and 23.0 months in patients on RVD-28 (p=0.492). Median PFS-2 (C1D1 to death or second progression) observed was 35.0 months in patients treated with RVD-21 and 24.6 months in patients treated with RVD-28 (p=0.404). No statistically significant difference was noted in overall response rate, with 72.1% patients in RVD-21 and 62.5% patients in RVD-28 achieving VGPR or better (p=0.442). Time to VGPR analysis did not yield a statistically significant difference between the two RVD schedules (2.3 months in RVD-21 and 2.8 months in RVD-28) (p= 0.104). For each outcome, baseline variables with p<0.1 in the univariate analyses were adjusted in the Cox regression models with RVD schedule as the independent variable. No significant associations were found between RVD schedule and OS, PFS-1, PFS-2 or time to VGPR. These findings were consistent with the univariate analyses. No statistically significant differences in the incidence of adverse events were found in patients in either of the treatment . Among the patients treated with RVD-21, 23.5% reported any grade of neuropathy compared to 16.7% of patients treated with RVD-28 (p=0.575) (Table 1).
Conclusion. No differences in efficacy and safety outcomes were found among the RVD-21 and RVD-28 treatment schedules supporting individualized approach to induction schedule based on patient's ability to tolerate therapy. Shorter therapy schedules in RVD-21 allow for faster time to transplant, however, the time to achieve VGPR was not different.
Hillengass: Beijing Medical Award Foundation: Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees; Skyline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; Axxess Network: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Curio Science: Speakers Bureau; Beijing Life Oasis Public Service Center: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal